Summary:
Argonne researchers produce trace amounts of hydrogen with visible light by merging light-collecting proteins from a single-celled organism with a graphene platform.
Both graphene and protein absorb the light and re-direct electrons towards the titanium dioxide. Electrons interact with protons at the site of the platinum nanoparticles to produce hydrogen.
In-Depth Story:
Researchers at the US Department of Energy’s (DOE) Argonne National Laboratory have created a small scale “hydrogen generator” that uses light and a two-dimensional graphene platform to boost production of the hard-to-make element.
The research also unveiled a previously unknown property of graphene. The two-dimensional chain of carbon atoms not only gives and receives electrons, but can also transfer them into another substance.
Hydrogen is virtually everywhere on the planet, but the element is typically bonded with other elements and must be separated from oxygen in H2O to produce free hydrogen. The commercial separation process uses natural gas to react with superheated steam to strip away hydrogen atoms producing hydrogen fuel, but also carbon dioxide —a greenhouse gas byproduct which escapes into the atmosphere.
Argonne’s early-stage generator, composed of many tiny assemblies, is proof that hydrogen can be produced without burning fossil fuels. The scale is small, a little smaller than the diameter of spider silk. Scaling this research up in the future may mean that you could replace the gas in your cars and generators with hydrogen—a greener option, because burning hydrogen fuel emits only water vapor.
“Many researchers are looking to inorganic materials for new sources of energy,” said Elena Rozhkova, chemist at Argonne’s Center for Nanoscale Materials, a DOE Office of Science (Office of Basic Energy Sciences) User Facility. “Our goal is to learn from the natural world and use its materials as building blocks for innovation.”
For Rozhkova, this particular building block is inspired by the function of an ancient protein known to turn light into energy. Researchers have long known that some single-celled organisms use a protein called bacteriorhodopsin (bR) to absorb sunlight and pump protons through a membrane, creating a form of chemical energy. They also know that water can be split into oxygen and hydrogen by combining these proteins with titanium dioxide and platinum and then exposing them to ultraviolet light.
There is just one downside: titanium dioxide only reacts in the presence of ultraviolet light, which makes up a mere four percent of the total solar spectrum. If the researchers wanted to power their generators with sunlight, they’d need to improve on that.
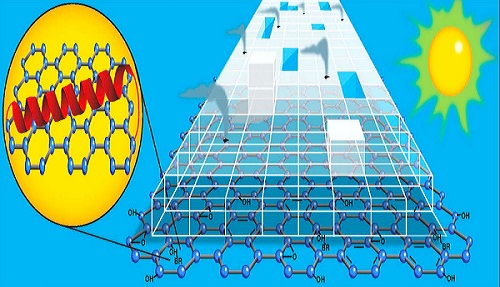
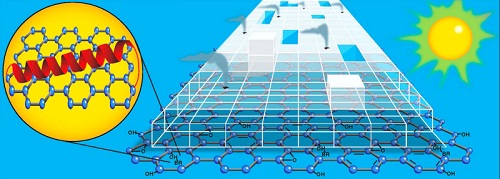
In order to produce greater amounts of hydrogen using visible light, the researchers looked for a new material. The new material would need enough surface area to move electrons across quickly and evenly and boost the overall electron transfer efficiency. The researchers also needed a platform on which biological components, like bR, could survive and connect with the titanium dioxide catalyst: in short, a material like graphene.
Graphene is a super strong, super light, near totally transparent sheet of carbon atoms and one of the best conductors of electricity ever discovered. Graphene owes its amazing properties to being two-dimensional.
“Graphene not only has all these amazing properties, but it is also ultra-thin and biologically inert,” said Rozhkova. “Its very presence allowed the other components to self-assemble around it, which totally changes how the electrons move throughout our system.”
Rozhkova’s mini-hydrogen generator works like this: both the bR protein and the graphene platform absorb visible light. Electrons from this reaction are transmitted to the titanium dioxide on which these two materials are anchored, making the titanium dioxide sensitive to visible light.
Simultaneously, light from the green end of the solar spectrum triggers the bR protein to begin pumping protons along its membrane. These protons make their way to the platinum nanoparticles which sit on top of the titanium dioxide. Hydrogen is produced by the interaction of the protons and electrons as they converge on the platinum.
Examinations using a technique called Electron Paramagnetic Resonance (EPR) and time-resolved spectroscopy at the Center for Nanoscale Materials verified the movements of the electrons within the system, while electrochemical studies confirmed the protons were transferred. Tests also revealed a new quirk of graphene behavior.
“The majority of the research out there states that graphene principally conducts and accepts electrons,” said Argonne postdoctoral researcher Peng Wang. “Our exploration using EPR allowed us to prove, experimentally, that graphene also injects electrons into other materials.”
Rozhkova’s hydrogen generator proves that nanotechnology, merged with biology, can create new sources of clean energy. Her team’s discovery may provide future consumers a biologically-inspired alternative to gasoline.
“These are the types of discoveries we can make at Argonne,” said Rozhkova. “Working in the basic energy sciences, we were able to demonstrate an energy-rich biologically-inspired alternative to gas.”
Publication Reference:
Peng Wang et al: Photoinduced Electron Transfer Pathways in Hydrogen-Evolving Reduced Graphene Oxide-Boosted Hybrid Nano-Bio Catalyst. ACS Nano, 2014.










Comments