The hybrid form of perovskite has properties that make it suitable for it to replace silicon (major component of conventional solar cells) to make highly efficient solar panels.
So researchers in Cambridge, Oxford and Munich have used perovskite materials to make high-brightness LEDs. This could one day make it possible for cheaper and easier ways to manufacture LED displays, a possibility that opens up a wide range of advantages for solar PV technology advancement.
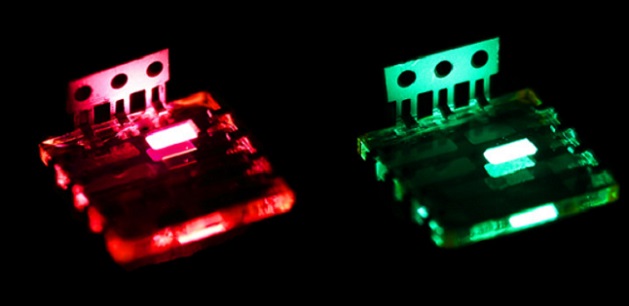
A hybrid form of perovskite – the same type of material which has recently been found to make highly efficient solar cells that could one day replace silicon – has been used to make low-cost, easily manufactured LEDs, potentially opening up a wide range of commercial applications in future, such as flexible colour displays.
This particular class of semiconducting perovskites have generated excitement in the solar cell field over the past several years, after Professor Henry Snaith’s group at Oxford University found them to be remarkably efficient at converting light to electricity. In just two short years, perovskite-based solar cells have reached efficiencies of nearly 20%, a level which took conventional silicon-based solar cells 20 years.
Now, researchers from the University of Cambridge, University of Oxford and the Ludwig-Maximilians-Universität in Munich have demonstrated a new application for perovskite materials, using them to make high-brightness LEDs. The results are published in the journal Nature Nanotechnology.
Perovskite is a general term used to describe a group of materials that have a distinctive crystal structure of cuboid and diamond shapes. They have long been of interest for their superconducting and ferroelectric properties. But in the past several years, their efficiency at converting light into electrical energy has opened up a wide range of potential applications.
The perovskites that were used to make the LEDs are known as organometal halide perovskites, and contain a mixture of lead, carbon-based ions and halogen ions known as halides. These materials dissolve well in common solvents, and assemble to form perovskite crystals when dried, making them cheap and simple to make.
“These organometal halide perovskites are remarkable semiconductors,” said Zhi-Kuang Tan, a PhD student at the University of Cambridge’s Cavendish Laboratory and the paper’s lead author. “We have designed the diode structure to confine electrical charges into a very thin layer of the perovskite, which sets up conditions for the electron-hole capture process to produce light emission.”
The perovskite LEDs are made using a simple and scalable process in which a perovskite solution is prepared and spin-coated onto the substrate. This process does not require high temperature heating steps or a high vacuum, and is therefore cheap to manufacture in a large scale. In contrast, conventional methods for manufacturing LEDs make the cost prohibitive for many large-area display applications.
“The big surprise to the semiconductor community is to find that such simple process methods still produce very clean semiconductor properties, without the need for the complex purification procedures required for traditional semiconductors such as silicon,” said Professor Sir Richard Friend of the Cavendish Laboratory, who has led this programme in Cambridge.
“It’s remarkable that this material can be easily tuned to emit light in a variety of colours, which makes it extremely useful for colour displays, lighting and optical communication applications,” said Tan. “This technology could provide a lot of value to the ever growing flat-panel display industry.”
The team is now looking to increase the efficiency of the LEDs and to use them for diode lasers, which are used in a range of scientific, medical and industrial applications, such as materials processing and medical equipment. The first commercially-available LED based on perovskite could be available within five years.
Publication Reference:
Zhi-Kuang Tan et al: Bright light-emitting diodes based on organometal halide perovskite. Nature Nanotechnology, 2014.










Comments