By looking at a piece of material in cross section, Washington University in St. Louis engineer Parag Banerjee, PhD, and his team discovered how to make what could be solar cells of the future.
Banerjee, assistant professor of materials science and an expert in working with nanomaterials, Fei Wu, graduate research assistant, and Yoon Myung, PhD, a postdoctoral research associate, also took a step toward making solar cells and more cost-effective.
Banerjee and his team worked with copper foil, a simple material similar to household aluminum foil. When most metals are heated, they form a thick metal oxide film.
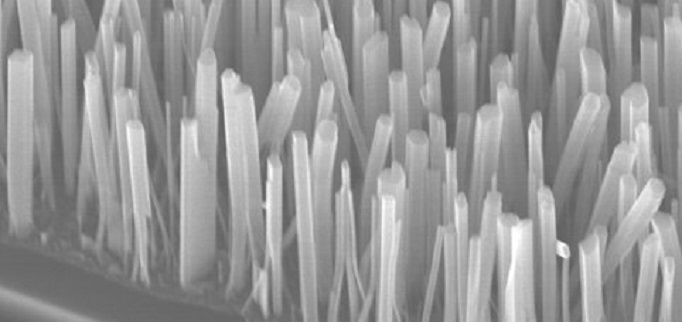
However, a few metals, such as copper, iron and zinc, grow grass-like structures known as nanowires, which are long, cylindrical structures a few hundred nanometers wide by many microns tall. They set out to determine how the nanowires grow.
“Other researchers look at these wires from the top down,” Banerjee says. “We wanted to do something different, so we broke our sample and looked at it from the side view to see if we got different information, and we did.”
Results of the research were recently published in CrystEngComm. Washington University’s International Center for Advanced Renewable Energy & Sustainability (I-CARES) and the McDonnell Academy Global Energy and Environment Partnership (MAGEEP) provided funding for the research.
The team used Raman spectroscopy, a technique that uses light from a laser beam to interact with molecular vibrations or other movements. They found an underlying thick film made up of two different copper oxides (CuO and Cu2O) that had narrow, vertical columns of grains running through them.
In between these columns, they found grain boundaries that acted as arteries through which the copper from the underlying layer was being pushed through when heat was applied, creating the nanowires.
“We’re now playing with this ionic transport mechanism, turning it on and off and seeing if we can get some different forms of wires,” says Banerjee, who runs the Laboratory for Emerging and Applied Nanomaterials (L.E.A.N.).
Like solar cells, the nanowires are single crystal in structure, or a continuous piece of material with no grain boundaries, Banerjee says.
“If we could take these and study some of the basic optical and electronic properties, we could potentially make solar cells,” he says. “In terms of optical properties, copper oxides are well-positioned to become a solar energy harvesting material.”
The find may also benefit other engineers who want to use single crystal oxides in scientific research. Manufacturing single crystal Cu2O for research is very expensive, Banerjee says, costing up to about $1,500 for one crystal.
“But if you can live with this form that’s a long wire instead of a small crystal, you can really use it to study basic scientific phenomena,” Banerjee says.
Banerjee’s team also is looking for other uses for the nanowires, including acting as a semiconductor between two materials, as a photocatalyst, a photovoltaic or an electrode for splitting water.
Reference:
Washington University in St. Louis












Comments