The sun doesn’t shine on the same spot all day, meaning solar panels produce energy intermittently. Solar energy researchers have been trying to store sunlight by splitting water into hydrogen and oxygen. A solar cell splits water with two electrodes, producing oxygen on one electrode, while generating hydrogen on the other.
Now a simple process made an electrode that absorbs sunlight and produces oxygen on tiny cobalt islands on a silicon electrode. Surprisingly, a crucial component was a silicon dioxide layer between the silicon and cobalt islands.
Using sunlight to create fuels (such as hydrogen on the other electrode in this solar cell) with inexpensively prepared electrodes may provide a solution to the intermittency drawback of solar energy.
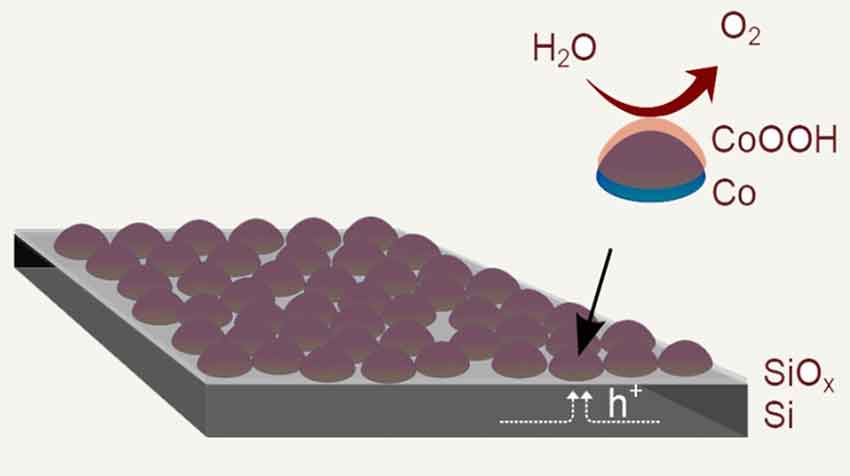
Image credit: Macmillan Publishers Ltd: Nature Materials, © 2015
Solar energy can be stored by using sunlight to split water (H2O) into hydrogen and oxygen. Speeding up half of the reaction at one of the solar cell electrodes, water-splitting catalysts, located on nano-sized cobalt (Co) islands, uses sunlight to drive the conversion of water to oxygen. Hydrogen is produced at the other electrode (not shown). This simple and inexpensive method produces a solar cell composed of a junction of metal (Co), insulator (SiOx), and a silicon semiconductor (Si) connected to an efficient water-splitting catalyst. The water-splitting catalyst is prepared by chemically combining the cobalt with oxygen (a process called oxidation), creating an outer shell of oxidized cobalt on the nano-islands.
To understand the important interfaces in a solar cell connected to a water-splitting catalyst, researchers from Missouri University of Science & Technology developed a method to simply and controllably produce such a system. A simple process called “electrochemical deposition” was used to place metal from a solution of ions onto the silicon surface.
The process modified the surface of a silicon electrode to facilitate the desired reactions and protect the underlying semiconductor. Advantageously, an interfacial oxide layer (SiOx) formed between the silicon and the deposited cobalt nano-islands.
This process produced a solar cell with a junction of metal (cobalt), a silicon-based insulator (SiOx), and a semiconductor. The scientists also found that the discontinuous nature of the nano-islands along with the interfacial oxide modified the electronic properties of the silicon surface and improved its solar performance.
Next the outer shell of the nano-islands was activated to achieve efficient water splitting. The activation involved chemically binding cobalt in the outer shell with oxygen to produce a shell of oxidized cobalt. With the metal-insulator-semiconductor solar cell directly connected to the water-splitting oxidized cobalt catalyst, water was efficiently split into oxygen with exposure to sunlight.
Therefore, in this solar cell that splits water, oxygen is generated on this new electrode, while hydrogen is generated on the other electrode. These efficient inexpensive solar cells may be the energy storage solution for intermittent solar energy.
Reference(s):
Publication: James C. Hill, Alan T. Landers, Jay A. Switzer. An electrodeposited inhomogeneous metal–insulator–semiconductor junction for efficient photoelectrochemical water oxidation. Nature Materials, 2015
Story: Simple Preparation for Affordable Solar Energy Storage. Department of Energy, Office of Science | June 28, 2016












Comments