- A new study tracked lithium metal deposition and removal from a battery anode while it was cycling to find clues as to how failure occurs.
- The research could help improve the use of pure lithium metal in anodes for batteries.
- It could also help reduce battery weight and dramatically extend duration.
Pure lithium metal is a promising replacement for the graphite-based anodes currently used in electric vehicle batteries.
It could tremendously reduce battery weights and dramatically extend the driving range of electric vehicles relative to existing technologies. But before lithium metal batteries can be used in cars, scientists must first figure out how to extend their lifetimes.
A new study led by Peter Khalifah — a chemist at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Stony Brook University — tracked lithium metal deposition and removal from a battery anode while it was cycling to find clues as to how failure occurs. The work is published in a special issue of the Journal of the Electrochemical Society honoring the contributions of Nobel Prize-winning battery researcher John Goodenough, who like Khalifah is a member of the Battery 500 Consortium research team.
“In a good battery, the rate of lithium plating (deposition) and stripping (removal) will be the same at all positions on the surface of electrodes,” Khalifah said. “Our results show that it’s harder to remove lithium at certain places, which means there are problems there. By identifying the cause of the problems, we can figure out how to get rid of them and make better batteries with higher capacities and longer lifetimes.”
Khalifah and his collaborators conducted the study using intense x-rays at the Advanced Photon Source, a DOE Office of Science user facility at DOE’s Argonne National Laboratory. They tracked lithium as it shuttled from cathode to anode and back during one complete charge and discharge cycle.
“The x-rays can see right through the battery and allow us to make many measurements very quickly to track what happens as the battery changes,” Khalifah said. “To the best of our knowledge, no one has ever been able to use x-rays to map lithium shuttling while it happens.”
One challenge: Lithium atoms are difficult to see using x-rays. The weak signal from the small number of lithium atoms that move between the cathode and anode can easily get obscured by stronger signals emitted by other materials that make up the battery — including the signal that would come from the large amount of lithium on a pure lithium metal anode.
To address that challenge, Khalifah’s team designed a battery cell using a “bare” anode — at least bare with respect to the presence of pre-existing lithium. This makes the signal of the shuttling lithium ions easier to measure. They then did a study comparing two different anode materials — copper and molybdenum — on which lithium ions were deposited as pure lithium metal after being extracted from the cathode material during operation of these batteries. This allowed the researchers to follow how uniformly lithium metal was added to and removed from anode surfaces. Comparing this process using copper and molybdenum anodes also offered an opportunity to identify differences between these two metals that might prove fruitful in designing improved batteries. Using this setup, the team mapped out how much lithium was present across the electrode while the cell was maintained at various stages of charge and discharge.
It took about an hour to collect maps with hundreds of data points. That mapping data could be used to identify changes that had occurred as a result of charging and discharging the battery, but the process of data collection was too slow to be useful for following the changes as they occurred. So, to track changes as they happened, the scientists used a more rapid data collection procedure to scan a small subset of 10 pixel-specific locations over and over again during battery cycling.
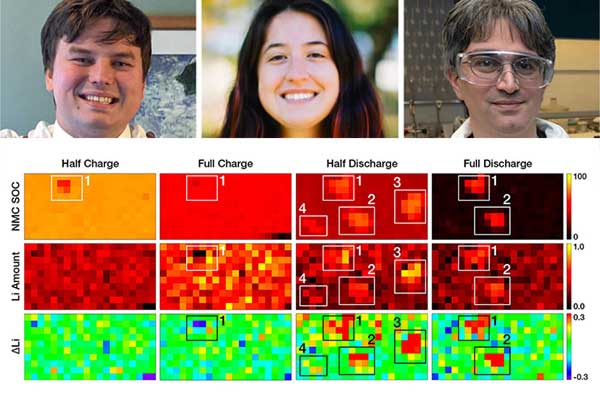
“We made the maps while the battery was in a resting state, starting at zero capacity, then took pixel measurements as we charged to half capacity. Then we stopped charging and made another map, then resumed pixel-specific measurements while charging to full capacity. We then discharged the cell while continuing to alternate mapping and pixel scans, stopping to collect maps at half discharge and full discharge,” Khalifah explained.
Results reveal variations
For the copper anode, all the points behaved as they should during charging: half the lithium capacity was deposited on the anode up to the half-charged state, and all possible lithium was deposited by the full charge state.
On discharge, large differences developed between pixels. In some pixels, the lithium was removed proportional to the discharge (half the lithium was stripped by the half discharge state, and all was gone by full discharge). Other pixels showed a lag in lithium removal, where stripping was slow during the first half of discharge, then sped up to complete the process by full discharge. In still other spots the lagging was so severe that most of the lithium remained on the anode even when the battery had been fully discharged.
“If the lithium is left behind, that reduces the capacity of the cell,” Khalifah said. “Each lithium atom left behind means one less electron flowing through the external circuit powered by the battery. You can’t extract all the capacity of the cell.”
The finding that these irregularities arose due to incomplete stripping of lithium was somewhat surprising. Prior to this study, many scientists had believed that lithium plating was the source of the worst problems in lithium metal batteries.
“In general, one expects it is more difficult to deposit lithium metal as the atoms have to be organized in the specific arrangement of the crystal structure of this metal,” Khalifah explained. “Removing lithium should be easier because any atom on the surface can be taken away without having to follow any specific pattern. Furthermore, if lithium is added more quickly than the atoms can be deposited homogenously across the surface, the growth tends to occur in the form of needle-like dendrites that can cause electrical shorts (and potentially fires) in the battery.”
The molybdenum anode showed a bit more variation during plating than copper, but less variation during stripping.
“Since the lithium behavior was better during the stripping step that caused the most overall irregularities in the anode, it implies that batteries using molybdenum foil substrates instead of copper substrates might yield higher capacity batteries,” Khalifah said.
However, it is not yet clear if the choice of metal is responsible for the better performance of the molybdenum anode. Another factor could be the distribution of electrolyte — the liquid through which the lithium ions travel as they shuttle back and forth between anode and cathode.
The mapping data showed that the regions of poor performance occurred in spots that were about five millimeters across. The size and shape of those spots and comparisons with other experiments suggest that poor spreading of the liquid electrolyte throughout the battery cell might be responsible for the local loss of capacity in those regions. If this is the case, Khalifah said, then the performance of the battery can likely be improved by finding a better method for distributing the electrolyte across the cathode.
“Follow-up experiments aimed at distinguishing between metal and solvent effects, and for testing the effectiveness of strategies for mitigating potential problems such as electrolyte inhomogeneity, will help advance the broader goal of developing high-capacity lithium metal anode batteries with long lifetimes,” Khalifah said.
—
Publication Referenced in the Article:
Monty R. Cosby, Gia M. Carignan, Zhuo Li, Corey M. Efaw, Charles C. Dickerson, Liang Yin, Yang Ren, Bin Li, Eric J. Dufek, Peter G. Khalifah. Operando Synchrotron Studies of Inhomogeneity during Anode-Free Plating of Li Metal in Pouch Cell Batteries. Journal of The Electrochemical Society, 2022; 169 (2): 020571 DOI: 10.1149/1945-7111/ac5345.
—
This article was written by the team at the DOE/Brookhaven National Laboratory.









Comments