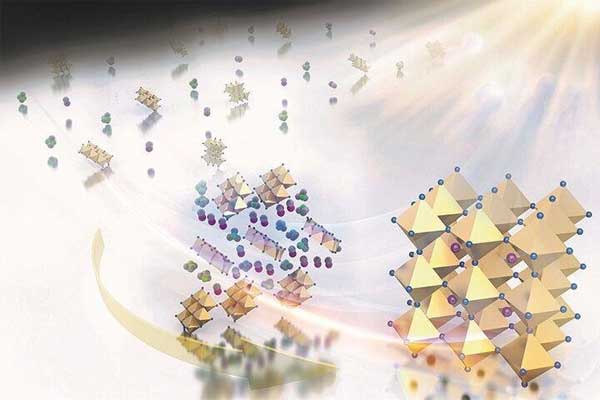
The work, published this month as the cover story for Nanoscale, offers a mechanism study of how a perovskite thin film changes its microscopic structure upon gentle heating, said Yan Yao, assistant professor of electrical and computer engineering and lead author on the paper. This information is crucial for designing a manufacturing process that can consistently produce high-efficiency solar panels.
Last year Yao and other researchers identified the crystal structure of the non-stoichiometric intermediate phase as the key element for high-efficiency perovskite solar cells. But what happened during the later thermal annealing step remained unclear. The work is fundamental science, Yao said, but critical for processing more efficient solar cells.
“Otherwise, it’s like a black box,” he said. “We know certain processing conditions are important, but we don’t know why.”
Other researchers involved with the project include first author Yaoguang Rong, previously a postdoctoral fellow at UH and now associate professor at Huazhong University of Science and Technology in China; UH postdoctoral fellows Swaminathan Venkatesan and Yanan Wang; Jiming Bao, associate professor of electrical and computer engineering at UH; Rui Guo and Wenzhi Li of Florida International University, and Zhiyong Fan of Hong Kong University of Science and Technology.
Yao is also a principal investigator at the Texas Center for Superconductivity at UH, which provided funding for the work.
The work also yielded a surprise: the materials showed a peak efficiency – the rate at which the material converted light to electricity – before the intermediate phase transformation was complete, suggesting a new way to produce the films to ensure maximum efficiency. Yao said researchers would have expected the highest efficiency to come after the material had been converted to 100 percent perovskite film. Instead, they discovered the best-performing solar devices were those for which conversion was stopped at 18 percent of the intermediate phase, before full conversion.
“We found that the phase composition and morphology of solvent engineered perovskite films are strongly dependent on the processing conditions and can significantly influence photovoltaic performance,” the researchers wrote. “The strong dependence on processing conditions is attributed to the molecular exchange kinetics between organic halide molecules and DMSO (dimethyl sulfoxide) coordinated in the intermediate phase.”
Perovskite compounds commonly are comprised of a hybrid organic-inorganic lead or tin halide-based material and have been pursued as potential materials for solar cells for several years. Yao said their advantages include the fact that the materials can work as very thin films – about 300 nanometers, compared with between 200 and 300 micrometers for silicon wafers, the most commonly used material for solar cells. Perovskite solar cells also can be produced by solution processing at temperatures below 150 degrees Centigrade (about 300 degrees Fahrenheit) making them relatively inexpensive to produce.
At their best, perovskite solar cells have an efficiency rate of about 22 percent, slightly lower than that of silicon (25 percent). But the cost of silicon solar cells is also dropping dramatically, and perovskite cells are unstable in air, quickly losing efficiency. They also usually contain lead, a toxin.
Still, Yao said, the materials hold great promise for the solar industry, even if they are unlikely to replace silicon entirely. Instead, he said, they could be used in conjunction with silicon, boosting efficiency to 30 percent or so.
Reference(s):
Publication: Yaoguang Rong, Swaminathan Venkatesan, Rui Guo, Yanan Wang, Jiming Bao, Wenzhi Li, Zhiyong Fan, Yan Yao. Critical kinetic control of non-stoichiometric intermediate phase transformation for efficient perovskite solar cells. Nanoscale, 2016
Research story: University of Houston | July 18, 2016 (source)









Comments