Lithium-ion batteries are a rapidly growing energy storage method due to their high energy density, especially in mobile applications such as personal electronics and electric cars. However, the materials currently used in Li-ion batteries are expensive, many of them, like lithium cobalt oxide (belonging to the EU Critical Raw Materials, CRMs), are difficult to handle and dispose of. Additionally, batteries using these materials have relatively short lifetimes.
New novel materials are being developed for next generation Li-ion batteries. One promising anode-cathode material pair is lithium titanate countered by lithium iron phosphate. The raw materials for these components are readily available; and they are safe to use, and easy to dispose of or recycle. And most importantly, batteries manufactured using these materials have significantly longer cycle and calendar lifetimes compared to the current battery technology.
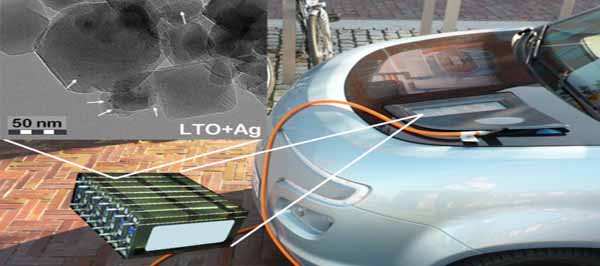
However, the main problem of these new materials is their low electric conductivity.
A study by University of Eastern Finland scientists opens up new electricity storage applications. The results were published recently in the Journal of Alloys and Compounds, which has a large audience especially in Asian countries, where most of the Li-ion battery manufacturing takes place currently.
“The electric conductivity problem can be solved by producing nanosized, high surface area crystalline materials, or by modifying the material composition with highly conductive dopants. We have succeeded in doing both for lithium titanate (LTO) in a simple, one-step gas phase process developed here at the UEF Fine Particle and Aerosol Technology Laboratory,” says Researcher Tommi Karhunen.
“The electrochemical performance of Li-ion batteries made out of the above mentioned material is very promising. The electrochemical properties were studied in collaboration with Professor Ulla Lassi’s group from Kokkola University Consortium Chydenius. The most important applications lie in batteries featuring, for example, fast charging required for electric buses, or high power needed for hybrid and electric vehicles,” says Professor Jorma Jokiniemi, Director of the Fine Particle and Aerosol Technology Laboratory.
Reference(s):
Publication: Tommi Karhunen, Juho Välikangas, Tiina Torvela, Anna Lähde, Ulla Lassi, Jorma Jokiniemi. Effect of doping and crystallite size on the electrochemical performance of Li4Ti5O12. Journal of Alloys and Compounds, 2016
Story: Novel synthesis method opens up new possibilities for utilizing Li-ion batteries | University of Eastern Finland — February 17, 2016











Comments